Bylvay has been studied in the largest phase 3 clinical trial program in cholestatic pruritus of ALGS and PFIC to date1,2
45
sites
15
countries
participated

PEDFIC 1 • 24 WEEKS1
Double-blind, randomized, placebo-controlled
Oral Capsules/Sprinkle
Once Daily
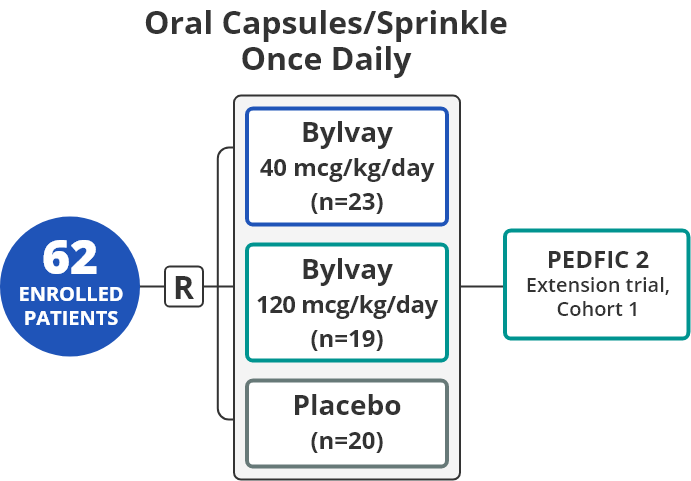
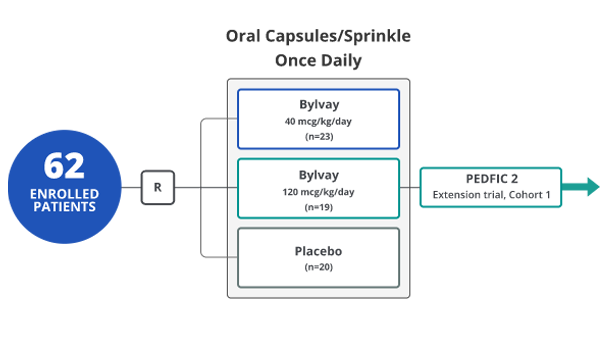
PEDFIC 1 participants had a confirmed diagnosis of
PFIC 1 or PFIC 2 and a history of significant pruritus1,5
PEDFIC 1 participants had a
confirmed diagnosis of PFIC 1 or
PFIC 2 and a history of significant
pruritus1,5
Co-primary Endpoints1,5
- Change in pruritus through week 24
- sBA response
Secondary Endpoints1,5
- Proportion of patients with positive pruritus assessments
every 4 weeks - Change in sleep parameters through week 24
PEDFIC 2 • 72 WEEKS1,3,4
Ongoing open-label extension
Bylvay 120 mcg/kg/day
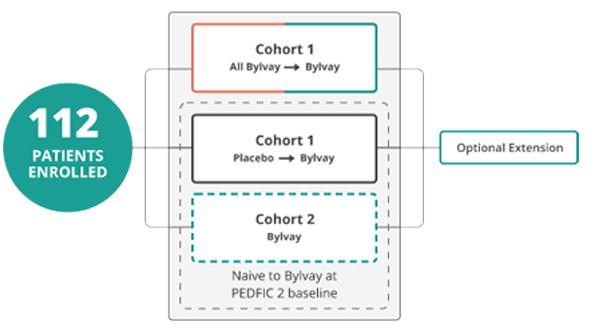
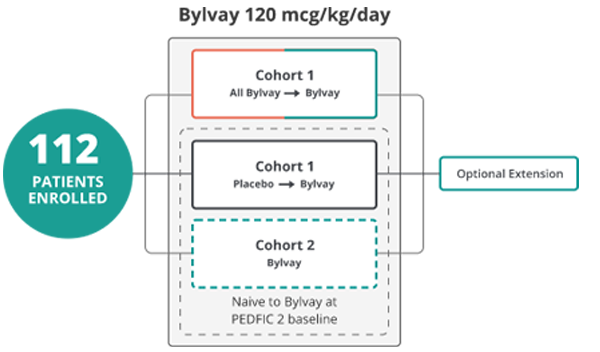
PEDFIC 2 includes a cohort continued from PEDFIC 1
and a second cohort open to any PFIC type4
Key Endpoints4,6
- Effect on pruritus
- Effect on sBA
- Proportion of patients with positive pruritus assessments
- Change in sleep parameters
Bylvay is FDA-approved for all PFIC types1
ALGS=Alagille syndrome; PFIC=progressive familial intrahepatic cholestasis; sBA=serum bile acid.
References:
- Bylvay Prescribing Information. Boston, MA: Albireo Pharma, Inc.; 2023.
- ClinicalTrials.gov. A double-blind, randomized, placebo-controlled, phase 3 study to demonstrate efficacy and safety of A4250 in children with progressive familial intrahepatic cholestasis types 1 and 2 (PEDFIC 1). NCT03566238. Updated September 5, 2021. Accessed April 23, 2023.
- Data on file PEDFIC 1 and 2 Figure Pru and sBA. 2022. Boston, MA: Albireo Pharma, Inc.
- Data on file A4250-008. Boston, MA: Albireo Pharma, Inc.
- Thompson RJ, Arnell H, Artan R, et al. Odevixibat treatment in progressive familial intrahepatic cholestasis: a randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2022;7:830-842.
- ClinicalTrials.gov. An open-label extension study to evaluate long-term efficacy and safety of A4250 in children with progressive familial intrahepatic cholestasis types 1 and 2 (PEDFIC 2). NCT03659916. Updated October 12, 2022. Accessed April 23, 2023.
- Bylvay Prescribing Information. Boston, MA: Albireo Pharma, Inc.; 2023.
- ClinicalTrials.gov. A double-blind, randomized,
placebo-controlled, phase 3 study to demonstrate
efficacy and safety of A4250 in children with
progressive familial intrahepatic cholestasis types 1
and 2 (PEDFIC 1). NCT03566238. Updated September
5, 2021. Accessed April 23, 2023. - Data on file PEDFIC 1 and 2 Figure Pru and sBA. 2022. Boston, MA: Albireo Pharma, Inc.
- Data on file A4250-008. Boston, MA: Albireo Pharma, Inc.
- Thompson RJ, Arnell H, Artan R, et al. Odevixibat treatment in progressive familial intrahepatic cholestasis: a randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2022;7:830-842.
- ClinicalTrials.gov. An open-label extension study to evaluate long-term efficacy and safety of A4250 in children with progressive familial intrahepatic cholestasis types 1 and 2 (PEDFIC 2). NCT03659916. Updated October 12, 2022. Accessed April 23, 2023.

